|
MEMBRANE POTENTIALS
 Membrane
Potentials and the Nerve Impulse Membrane
Potentials and the Nerve Impulse
The means
by which nerves conduct impulses have been puzzling researchers
for centuries. The electrical nature of the impulse was first
suspected by Luigi Galvani in 1780, when he caused the leg of a
frog to contract after stimulating it with an electric charge
from the newly developed Leyden jar. In the nineteenth century,
Emil Du Bois Reymond first demonstrated the action potential and
later wrote, "If I do not greatly deceive myself, I have
succeeded in realizing (albeit under a slightly different
aspect) the hundred years dream of physicists and physiologists,
to wit, the identity of the nervous principle with electricity."
Later, in 1902, Julius Bernstein postulated the "membrane
theory" of the nerve impulse, when he proposed that the impulse
is related to changes in the ion permeability of the membrane.
Finally much of our present knowledge concerning the events
associated with the action potential and the nerve impulse is
based on the ingenious work with the giant axon of the squid
performed by Hodgkin and Huxley in England and Curtis and Cole
in the United States during the late 1940s and early 1950s.
 OVERVIEW OF NEURON ACTIVITY
OVERVIEW OF NEURON ACTIVITY
Neurons are
ideally suited to function as the information-carrying units of
the nervous system. The length of their individual processes
varies from a fraction of a millimeter in the brain to axons
over 1 m in length in the spinal cord and peripheral nerves. The
information-carrying signal that travels along the neuron is an
electrical event called the impulse. All impulses which a neuron
conducts are nearly alike. Therefore the information which a
neuron can transmit is determined by the firing pattern as well
as the number of impulses per second (IPS) it sends. Neurons can
vary their impulse firing rates from 0 to just over 1000 IPS.
Because neurons have such a wide range of firing rates and
patterns they can transmit considerably more information to the
brain than they could if all they had was a simple "on-off"
system.
For those
functions in which speed of action is biologically important,
neurons with high conduction velocities are often employed.
Neurons with considerably slower conduction velocities are often
found in neural circuits which do not require such speed.
Conduction velocity is an inherent property of the neuron,
increasing with fiber diameter and the degree of myelination. In
mammalian neurons, conduction velocities vary anywhere from 0.2
up to 120 m/s.
Nervous
systems are incredibly complex networks of nerve cells in which
impulses traveling along one neuron initiate impulses in other
neurons at chemically responsive junctures called synapses.
Chemicals called neurotransmitters are released at these
synapses in response to the "arrival of impulses at the
presynaptic terminals of the first neuron.
When
impulses arrive at a sufficient number of these presynaptic
terminals, enough neurotransmitter is released to stimulate the
postsynaptic neuron to its excitation threshold. When this
happens there occurs on the membrane of the postsynaptic neuron
a rapid and reversible change called an action potential. Once
initiated this action potential generates a small local current
which initiates a second action potential on the adjacent
membrane segment. The local current from this action potential
will, in turn, initiate a third, and so on down the entire
length of the axon to the very ends of its terminal branches.
Although the action potential is actually reinitiated by this
series of events, we generally speak as though its propagation
is a continuous smooth process. This series of propagated action
potentials constitutes the impulse, and represents the signal
which forms the basis for the information which the nervous
system conducts.
 BASIC ELECTRICITY AND THE NEURON
BASIC ELECTRICITY AND THE NEURON
When
neurons conduct impulses, electrical currents flow through their
membranes and it is therefore not possible to understand the
former without a working knowledge of the latter. Besides,
electronic instruments are used to record action potentials and
impulses, and neurophysiologists commonly employ electrical
terms and symbols in describing neuronal events. So it is
actually well worth to review a few basic principles of
electricity which are critical to the understanding of nerve
cells.
 Current
Current
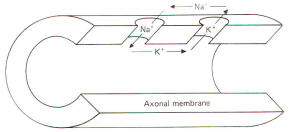 |
Current is carried in wires by electrons, but in
biological systems such as the neuron it is carried by
ions. The passage of 6X1018 electrons or
monovalent ions past any cross section of a conductor
represents an electric charge equal to one coulomb (C).
Current I represents the rate of flow of electric
charge. Its basic unit is the ampere (A), which
represents the flow of one coloumb per second. Each mole
of monovalent ion can transfer 96.500 C of electric
charge. a value useful to the neurophysiologist called
the Faraday constant. By convention in biological
systems, current is pictured as flowing in the direction
of the positive ions (Fig-1).
Because of relatively high extracellular and low
intracellular concentrations. current flow outside the
nerve cell and inward through the membrane is primarily
carried by Na+ ions. Similarly, intracellular
and outward currents are primarily carried by K+
ions because of its relatively high intracellular and
low extracellular concentrations. |
|
Fig-1 |
 Resistance and Conductance
Resistance and Conductance
All
conducting media offer some degree of resistance to the passage
of current whether carried by electrons or by ions. The unit of
resistance R is the ohm (Ω).
It represents the resistance of a conductor such that a constant
current of one ampere requires a potential of one volt between
its ends. All things being equal, current follows the path of
least resistance in any circuit. Neurophysiologists also use a
related value called conductance g. It represents the reciprocal
of resistance. The unit of conductance is the siemen (S).
However, the earlier term mho (ohm spelled backward) is commonly
used in most of the classical literature. Because of this
reciprocal relationship, all statements concerning resistance
are reciprocally related to conductance.
g=1/R
where
g
= conductance, S and R =
resistance, Ω
Concerning resistance are
reciprocally related to conductance.
Neurophysiologists are often
concerned with the total resistance in several resistive
elements. Neuronal membranes behave in part as if they were
composed of parallel resistive elements, while the extracellular
and intracellular fluids surrounding the membrane behave like
series resistors. Since electric currents flow through the
membrane and both of these fluids during impulse conduction, it
is important to be able to estimate the total resistance
involved. Biologically, the important point to remember here is
that the total resistance of series resistors is equal to their
sum, but the total resistance of parallel resistors is equal to
a value less than their sum. Fig-2 illustrates the series
resistive nature of the axoplasm and extracellular fluid, while
Fig-3 pictures the axonal membrane in the form of parallel
resistors.
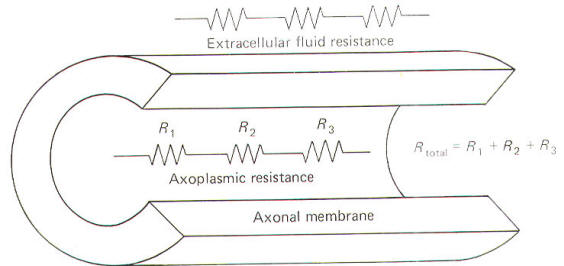 |
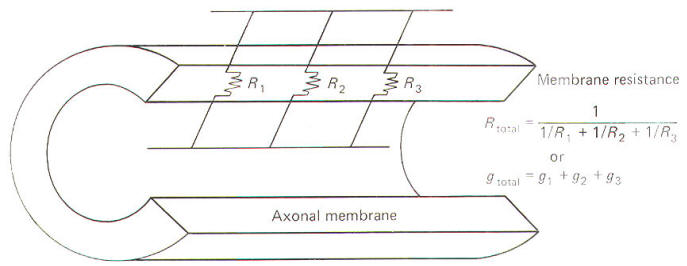 |
|
Fig-2 |
Fig-3 |
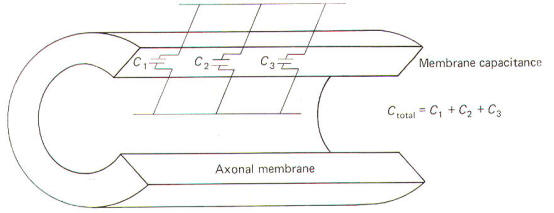
 Capacitance
Capacitance
The
neuronal membrane behaves in part as if it were composed of
parallel capacitors. A capacitor is a charge-storing component
consisting of two conductors separated by a dielectric
(insulator). The membrane represents the dielectric while the
extracellular fluid and the axoplasm represent the conductors
(Fig-4).
The unit of
capacitance C is the farad (F). It represents the capacitance of
a capacitor in which a charge of one coloumb produces a
potential difference of one volt between the terminals
(conductors). The relationship between capacitance (in farads)
and the potential difference (in volts) produced by a given
charge separation (in coulombs) is given by
C= Q/V where C =
capacitance, F Q = charge, C V = potential, V
By
manipulation of the equation we can see that the charge that
needs to be separated in order to produce a specific voltage is
given by:
Q=CV
Similarly,
the potential developed by the transfer of a given charge across
a capacitance is given by:
V= Q/C
Neurophysiologists are generally concerned with the total
capacitance in a section of membrane. Since the neuronal
capacitance is only associated with the membrane, we need not
concern ourselves with other aspects of the neuron, Because the
membrane behaves as if it were in part composed of parallel
capacitors, we are interested in the rules governing parallel
capacitors (Fig-5).
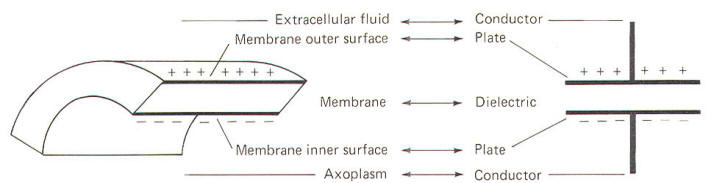 |
 |
| Fig-4 |
Fig-5 |
 Electrical Potential and Ohm's Law
Electrical Potential and Ohm's Law
The unit of
potential E is the volt (V). The difference in potential between
two points is related to the work done in moving a point charge
from the first point to the second. It is equal to the
difference in the value of the potentials at the respective
points. Biologically important voltages are usually quite small,
in the order of millivolts (mV) or microvolts (µV).
In simple
direct current (dc) wire circuits, the battery is an electronic
component which represents a potential difference as well as a
source of charge (electrons). In neurons, ions represent the
charge while the chemical (concentration) gradient for a given
ionic type represents the potential for that ion. The
relationship between potential, current, and resistance is
expressed by Ohm's law:
I=E/R
where I = current, A E = potential, V R
= resistance, Ω
In neuron
studies we are often concerned with conductance. Consequently a
useful form of the equation is:
I
=Eg
where g = conductance, S
 Resistance and Capacitance (RC)
Circuits
Resistance and Capacitance (RC)
Circuits
Functionally a capacitor can do three things. It can become
charged, it can store a charge, and it can discharge. When a
capacitor is connected to a voltage source, current will flow
and build up charges on one side of the capacitor while removing
them from the other side in the process of completing the
circuit. Current will flow in the circuit only until the charge
on the capacitor attains the same potential as the voltage
source. At the point the capacitor is fully charged, current
will no longer flow in the circuit. A resistor is usually
pictured as being in series with the capacitor in such a circuit
- hence, the name resistance-capacitance (RC)
circuit.
If the
voltage source is removed and the charged capacitor and resistor
are connected in a closed loop, current will once again flow as
charges are drawn off the capacitor through the resistor to
equalize on both sides of the dielectric. Thus the capacitor is
discharged and the potential removed. This current which flows
only when the capacitor is being charged or discharged is called
capacitive current Ic . It is proportional to the
rate of change of voltage across the capacitor.
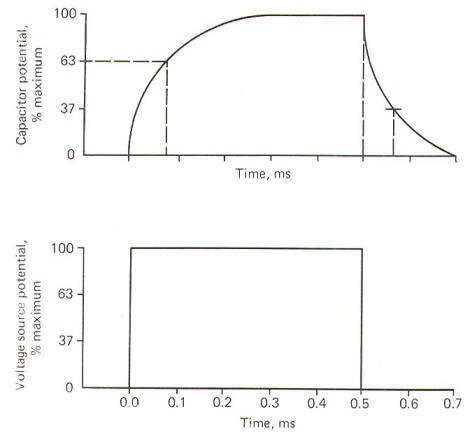
All
physical systems require a certain amount of time to transmit
given quantities of charge from input to output. This time in
simple RC systems is characterized by the system time constant
t. It is mathematically equal
to the product of the resistance (in ohms) and the capacitance
(in farads). The resultant time constant is in seconds and
represents the time required for the voltage to reach 1 -1/e (63
percent) of its final value. The symbol e is the base of the
natural logarithm (2.71828"').
t=RC
where t = time constant, s R = resistance,
Ω C =
capacitance, F
When a
voltage source is suddenly applied across an uncharged RC
circuit, there is a delay in the rise of the potential developed
on the capacitor, which is accounted for by the time required to
store charges (Fig-6). The time constant represents the time in
seconds it takes for the capacitor to produce a voltage 63
percent as high as its final value when it is fully charged.
Similarly, when the capacitor is discharged, it takes just as
long (i.e., one time constant) for the capacitor to lose 63
percent of its charge.
 |
| Fig-6: |
 THE RESTING MEMBRANE POTENTIAL
THE RESTING MEMBRANE POTENTIAL
All cells
exhibit an electrical potential across their membranes called a
membrane potential (MP). However, nerve and muscle cells are
somewhat unique in that this membrane potential can be reduced
(depolarized) or increased (hyperpolarized) as a result of
synaptic activity. This feature makes nerve and muscle cells
excitable.
When a
neuron is not being stimulated its membrane potential is
relatively stable and is therefore referred to as a resting
membrane potential (RMP). A typical RMP for mammalian nerve and
muscle cells lies between 70 and 100 mV, with the intracellular
fluid negative. For illustrative purposes consider a common
average for a large mammalian nerve cell axon of -85 mV with the
dendritic zone (soma and dendrites) being less polarized at
approximately -70 mV.
Much of
what we believe today concerning membrane potentials and action
potentials is based on experiments with the giant axon of the
squid. Such studies laid the groundwork for almost all of our
present assumptions concerning nerve excitability. Accordingly,
many of the examples included here will be based on action
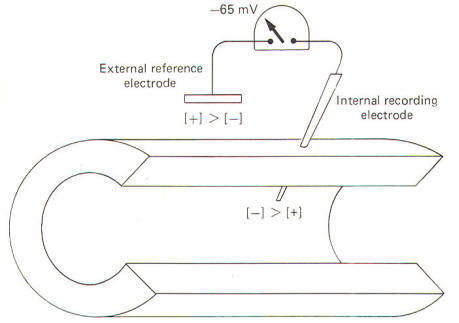
potentials and impulses in the squid axon. When a squid or
mammalian nerve axon is penetrated by a recording micro
electrode and the internal potential compared to an external
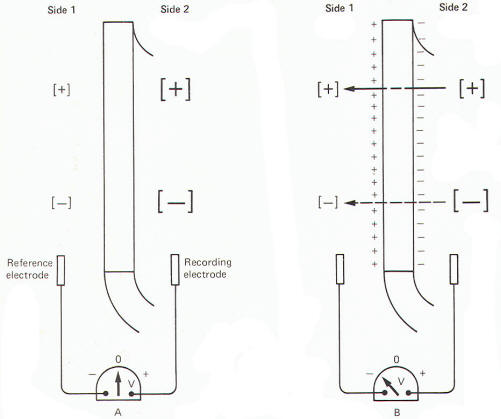
reference electrode, the axoplasm is found to be negative with
respect to the outside. The magnitude of this potential is about
-65 mV in the squid. The obvious assumption which can be made is
that there are slightly more positive than negative charges
outside, and slightly more negative than positive charges inside
(Fig-7).
 |
| Fig-7 |
How
important is it for nerve cells to have a resting membrane
potential?
Quite
simply, without it (1) they would not be excitable, (2) they
could not produce action potentials, and (3) they could not
conduct impulses. Thus because of its important role in the
impulse conducting process, a good place to start our discussion
is with the origin of the RMP itself.
 Ionic Distribution and the Resting
Membrane Potential
Ionic Distribution and the Resting
Membrane Potential
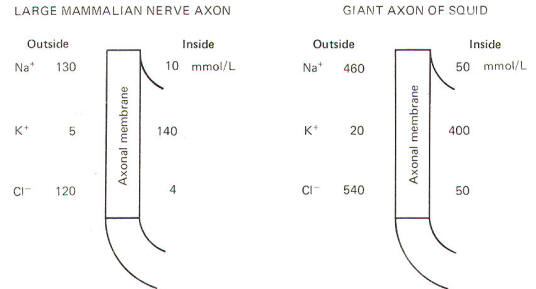
Julius
Bernstein demonstrated in the early 1900s that ionic fluxes
across the membrane were important to the impulse-conducting
capabilities of neurons. He demonstrated that resting membranes
were typically more permeable to K+ ions than they
were to Na+ ions. Typical extracellular and
intracellular concentrations of Na+, K+,
and Cl- in a large mammalian nerve cell axon and the
giant axon of the squid are illustrated in Fig-8.
 |
 |
| Fig-8 |
Fig-9 |
Even a
quick observation of the various concentrations in Fig-8 shows
us that Na+ and Cl- are much more
concentrated extracellularly, while K+ is much more
concentrated intracellularly. The membrane which separates these
two solutions in both the squid and mammalian cells is freely
permeable to water but is much less permeable to the
above-listed ions. Not depicted in Fig-8 are the nonpermeable
anions found within the intracellular fluid. These anions are
composed primarily of large protein molecules. Because of the
free permeability of the membrane to water, the inside and
outside solutions have virtually the same osmolality.
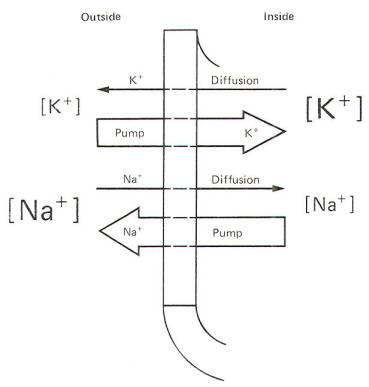
Even though
the membrane is not very permeable to either cation listed in
Fig-8, recall that Bernstein showed the resting membrane to be
more permeable to K+ than to Na+. It was
subsequently discovered that CI- permeates more
readily than K+ in the mammalian axon and less
readily than K+ in the giant axon of the squid. The
membranes of both species actively transport Na+ to
the outside and K+ to the inside. Hence the tendency
for the ions to diffuse down their chemical gradients is
counterbalanced by the Na+ and K+ active
transport system (Na+/K+ "pump")
transporting these ions against their chemical gradients
(Fig-9).
 The Principles of Equimolality and
Electrical Neutrality
The Principles of Equimolality and
Electrical Neutrality
When a cell
is at rest it obeys two basic principles, the principle of
equimolality and the principle of electrical neutrality. These
two principles are summarized here.
1 The
principle of equimolality. The concentrations of osmotically
active particles on both sides of the cell membrane should be
approximately equal.
2 The principle of electrical neutrality. The number of
extracellular cations and anions should be approximately equal.
Similarly, the number of intracellular cations and anions should
be approximately equal.
Chemical
analysis of the solutions on each side of the two types of nerve
cells verifies that these two principles are essentially true. A
second examination of the ionic distributions will show that the
high extracellular concentration of Na+ is primarily
balanced by the high extracellular concentration of Cl-,
while the high intracellular K+ concentration is
primarily balanced by the high intracellular concentration of
large nonpermeating anions we referred to earlier.
We have
neglected to list other such ions as Mg2+ , Ca2+,
and several others which are also present in the solutions on
either side. Their concentrations are small and otherwise
unimportant in the events of the action potential and impulse.
Nevertheless they help to contribute to the principles of
equimolality and electrical neutrality.
You might
well be wondering how it is possible to have a resting membrane
potential if the solutions on both sides of the membrane are
electrically neutral. The answer lies in the fact that the
principle of electrical neutrality is only approximately true.
As we have previously noted (Fig-7) there are actually slightly
more cationic than anionic charges outside and slightly more
anionic than cationic charges inside. This slight imbalance, or
violation of the principle of electrical neutrality, is
sufficient to produce the potential difference across the
resting membrane. As we will see later an imbalance of only a
few picomoles (10-12 mol) is sufficient to produce
the resting membrane potential
 Ionic Diffusion and the Resting
Membrane Potential
Ionic Diffusion and the Resting
Membrane Potential
A
relatively simple experiment might be helpful in understanding
how the resting membrane potential develops. If you were to
place a highly concentrated salt solution on one side of a
selectively permeable membrane and a less concentrated solution
on the other side, the salt would diffuse through the membrane
from the highly concentrated to the less concentrated side until
it reached equilibrium. Now if the membrane was more permeable
to the cation of the salt than it was to the anion, positive
charges would migrate to one side faster than negative charges
and a charge separation would develop across the membrane with
one side more positive than the other (Fig-10). A sensitive
voltmeter applied across the membrane would register a potential
difference, and because this potential is caused by unequal
diffusion rates it can properly be called a diffusion potential.
 |
| Fig-10 |
The resting
membrane potential which exists in both the mammalian and squid
axons is thought to be primarily due to a diffusion potential
caused by the charge separation which results as K+
ions diffuse outward down their concentration gradient, leaving
the large nonpermeating anions behind.
 The Nernst Equation and the Equilibrium
Potential
The Nernst Equation and the Equilibrium
Potential
As K+
ions diffuse outward following their chemical (concentration)
gradient, the outside of the membrane becomes increasingly
positive while the inside becomes more negative. This continual
tendency for K+ to diffuse outward is increasingly
opposed by the buildup of an electrical gradient in the opposite
direction, from outside to inside. That is, the increasing
positivity on the outside opposes the further flow of positively
charged K+ outward, and the increasing negativity of
the inside surface of the membrane tends to restrict the escape
of K+. When the electrical gradient has increased to
a point where it is sufficient to stop the net outward flow of K+,
the ion is said to be at electrochemical equilibrium.
The
relationship between the concentration gradient across the
membrane of any given ion and the membrane potential which will
just balance it at electrochemical equilibrium is given by the
physiochemical relationship known as the Nernst equation. It
represents the equilibrium potential for that particular ionic
type. The Nernst equation is commonly used in one of two forms.
The second equation, derived from the first, is often preferred
because it is easier to work with and suffers little loss in
accuracy.
E= RT/zF InC1/C2
(2-1)
E= 58 logC1/C2
(2-2)
where E
= equilibrium potential [expressed in volts in Eq. (2-1) and
millivolts in Eq. (2-2)]. R = universal gas constant,
8.32 J mol-1 K-1 . z =
valence and charge of ion. F = Faraday constant, 96,500
C/mol. C1/C2=
chemical gradient. In= natural logarithm. log= common logarithm.
61 is used
if preparation is at body temperature (37oC);
58 is used if preparation is at room temperature
(20oC).
The equilibrium potentials calculated by these equations are
slightly different for each nerve cell depending on whether Eq.
(2-1) or Eq. (2-2) is used. This is due to rounding errors in
converting the quantity (RT/ zF) In to the quantity 61
log or 58 log. However, for all practical purposes, the error is
quite small and can be ignored.
The Nernst
equation is interpreted this way. In the squid axon, K+
is 20 times more concentrated inside than outside and therefore
has a chemical gradient directed outward. It would require an
external positivity of about 76 mV (internal negativity equal to
-76 mV) to just balance this gradient at electrochemical
equilibrium and stop the net diffusion of K+ outward.
Since the RMP of the squid axon isn't quite this negative
inside, there is a continual tendency for K+ to
diffuse outward. Now consider that a cation gradient from inside
to outside and an anion gradient from outside to inside will be
balanced at electrochemical equilibrium by internal negativity.
Similarly, a cation gradient from outside to inside and an anion
gradient from inside to outside will be balanced by internal
positivity. Consequently, the following forms of the Nernst
equation can theoretically predict both the magnitude and
polarity of the internal potential:
E =
- 58 log (cationi/cationo)
E =
-58 log (aniono/anioni)
 Sodium and Potassium Equilibrium
Potentials
Sodium and Potassium Equilibrium
Potentials
The
equilibrium potential necessary to just balance a given chemical
gradient can be theoretically predicted by the Nernst equation.
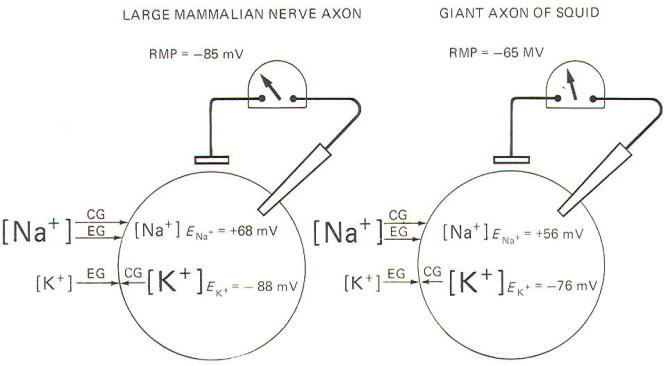
The values for the mammalian nerve cell and the squid axon are
listed below.
Large
mammalian nerve cell ENa+ = 68 mV
EK+ = -88 mV
Giant axon
of the squid ENa+ = 56 mV
EK+ = -76 mV
Remember
that the RMP of the large mammalian nerve cell is -85 mV,
while it is about -65 mV in the giant axon of the squid. Since
the equilibrium potentials for K+
and Na+
listed above are
not the same as the resting membrane potentials, it follows that
neither K+
nor Na+
is really at electrochemical
equilibrium in the large mammalian nerve cell nor in the giant
axon of the squid. Any time that an ion is not in electrochemical
equilibrium, net diffusion of that ion will occur and the
chemical gradient will change unless some other factor such as
membrane active transport acts to restore the gradient. Of
course, in the nerve cells described here, the Na+/K+
pump
does just that, and their respective chemical gradients are
maintained.
 Electrochemical Equilibrium and the Resting Membrane Potential
Electrochemical Equilibrium and the Resting Membrane Potential
Neither Na+
nor K+
is in electrochemical equilibrium across the resting
membrane of the mammalian neuron and the giant axon of the squid
(Fig-11).
 |
| Fig-11 |
For Na+,
notice that both the chemical and electrical gradients are
directed inward. In order to be in equilibrium, the inside would
need to be about +68 mV in the mammalian neuron, and about +56 mV
in the squid. And we know by intracellular
microelectrode recording that both interiors are actually
negative in the resting membrane.
In the case
of K+
ions, the electrical gradient is directed inward while
the chemical gradient is directed outward because of relatively
high intracellular K+
concentration. The inside would need to
be about -88 mV in the mammalian neuron and about -76 mV in the
squid in order for K+
to be in electrochemical equilibrium.
Notice that the experimentally measured RMP is very close to
these values in each case. That is, EK+ = -88 mV compared to an
RMP of -85 mV in the large mammalian nerve axon, and EK+= -76 mV compared to an RMP of -65 mV in the giant axon of the squid.
It is apparent that K+
is almost in electrochemical equilibrium
across the resting membrane of both cells. Nevertheless, the
respective potassium equilibrium potentials are slightly more
negative than their resting membrane potentials. Consequently,
there is a continual tendency for K+
ions to diffuse outward.
 The Ionic
Imbalance of Sodium and Potassium
The Ionic
Imbalance of Sodium and Potassium
You might
be wondering at this point how the Na+/K+
"pump" fits into the
picture. Earlier, we noted that both the
electrical and chemical gradients for sodium are directed
inward. In addition, while the membrane is not easily penetrated
by Na+, some ions will nevertheless cross. Why then doesn't Na+
simply diffuse down its two gradients and reach equilibrium on
each side of the membrane? The answer lies in the capability of
the cell membrane to actively transport (pump) Na+
outward,
against these two gradients.
Not all of
this actively transported Na+
stays outside, however, since a
small amount leaks back inward because of the slight
permeability of the membrane to this ion. One can readily
appreciate, however, that the outward Na+
transport and the
inward Na+
diffusion must match each other in effectiveness
since there is no net change in the extracellular and
intracellular concentrations of this ion during the time that
the membrane is in the resting state.
As far as K+
is concerned, remember that the chemical gradient is outward
while the electrical gradient is inward. This inward electrical
gradient coupled with the fact that the membrane actively
transports K+
to the inside accounts for the high intracellular
K+
concentration found in both cell types. Once again, not all
of the actively transported K+
which is pumped inward stays
inside. Because of the outward-directed chemical gradient and
the limited permeability of the membrane to this ion, some K+
diffuses outward.
The
membrane of the resting mammalian nerve axon is typically 100
times more permeable to K+
than to Na+, while in the squid axon
a 25:1 ratio is observed. Nevertheless, the inward pumping and
outward diffusion of K+
must once again match each other since
there is no net change in the inside and outside concentrations
of this ion during the time the membrane is in the resting
state.
 The
Goldman-Hodgkin-Katz Equation
The
Goldman-Hodgkin-Katz Equation
In the resting membrane, none of the cations
and anions in the solutions on either side of the membrane are
at electrochemical equilibrium. Consequently, they are diffusing
across the membrane with different diffusion rates and in
different directions at all times in the resting membrane.
The only time an ion won't diffuse is when (1) it is
at electrochemical equilibrium or (2) the membrane is not
permeable to it at all. Consequently a variety of charge
separations are occurring simultaneously across the membrane,
with each contributing to a greater or lesser extent to the
experimentally measured resting membrane potential.
Hodgkin and
Katz, using a formula developed earlier by Goldman, attempted
to theoretically predict the resting membrane potential by
considering the combined effects of all these ions including (1)
the ionic charge, (2) the direction of the chemical gradient,
and (3) the relative permeability of the membrane to each.
VM
= -58 log { [Na+]i PNa+ + [K+]i
PK+ + [CI-]o PCl-
}/{[Na+]o PNa+ + [K+]o
PK+ + [CI-]i
PCl-}
where VM =
membrane potential, mV. Pion =
membrane permeability for a given ion.
The accuracy of this equation in predicting
actual resting membrane potentials is dependent on the
permeability factors for each ion which are only close
approximations of their true values. Nevertheless, the
predicted values are usually quite close to the measured RMPs.
Careful
examination of this equation will show several things. First
notice that the Goldman-Hodgkin-Katz equation is an extension of
the Nernst equation. Since it considers the collective
contributions of Na+, K+, and CI- chemical gradients as well
as the relative permeability of the membrane to each, the
integrated equilibrium potential which the equation predicts is
at least theoretically a close approximation of the RMP itself.
The theoretical prediction which this equation makes for the RMP
of the large mammalian nerve cell at body temperature and the
giant axon of the squid at room temperature is given below.
Large
mammalian nerve cell
VM = -61
log (0.01) +1400(1) + 120(2)/ 130(0.01)+ 5(1) + 4(2) = -87 mV
Giant axon
of the squid
VM
= - log 50(0.04) +
400(1) + 540(0.45) /460(0.04) + 20(1) + 50(0.45) =-59 mV
 THE ACTION
POTENTIAL AND THE IMPULSE
THE ACTION
POTENTIAL AND THE IMPULSE
Earlier we
noted that when a single area of axonal membrane is stimulated
it becomes excited and undergoes a rapid and reversible
electrical change called an action potential. And recall further
that this action potential propagates as a continuous impulse
down the entire length of the axon. Let's now examine the
changes which occur in the neuron during the action potential.
The action
potential results from a sudden change in the resting membrane
potential (a condition necessary for impulse conduction). To
illustrate this point, it is usually convenient in experimental
laboratory conditions to stimulate the neuron at some point on
its axon. You should recognize that this is not a normal
situation. Neurons are rarely stimulated on their axons in vivo.
Instead they are stimulated to produce action potentials in
vivo via (1) generator potentials from sensory receptors, (2)
neurotransmitters from presynaptic terminals at synapses, and
(3) local currents. Nevertheless, an action potential is still
an action potential no matter where or how it is produced and
the axon is generally much more accessible in experimental
situations than is the rest of the neuron.
By this
time you should be aware that the resting membrane is a
polarized membrane. That is, unlike charges are separated at the
membrane with the inside negative and the outside positive.
When the membrane is stimulated by an electronic stimulator in
the laboratory, its resting membrane potential begins to
decrease. That is, it becomes less negative and hence less
polarized. If it is depolarized to a critical level known as the
excitation threshold, an action potential will be produced at
the point of stimulation. Once the membrane potential is
depolarized to the excitation threshold, its Na+
channels
(routes through which Na+
ions cross the membrane) suddenly open
and a tremendous increase in Na+
conductance g",,+ occurs with
Na+
ions now free to diffuse down both their chemical and
electrical gradients. This is called sodium activation. You
should note that conductance
g
is the electrical analog of permeability P. Thus
it is also appropriate to say that there is a sudden and marked
increase in sodium permeability on the part of the membrane
when the excitation threshold is reached.
As the
positively charged Na+
ions suddenly diffuse inward the RMP is
greatly disturbed at the local site of stimulation. Sufficient
positively charged Na+
is removed from the immediate membrane
exterior surface and transferred to the immediate membrane
interior surface to totally eliminate the internal negativity
and replace it with positivity. Measurement now would record a
reversed potential showing the interior now positive with
respect to the exterior. We will see later that only a few
picomoles of Na+
actually need to diffuse inward to change the
membrane potential by 125 mV, that is, from a RMP of -85 mV to a
reversed potential of +40 mV in the large mammalian neuron or a
RMP of -65 mV to a reversed potential of +55 mV in the giant
axon of the squid.
This local
reversed potential is not allowed to last. Even before the
intracellular fluid reaches its maximum positivity, the local
membrane channels for K+
open, causing a great increase in
membrane permeability to K+
with a resulting increase in gK+
and carrying positive charges to the outside down their chemical
and electrical gradients. At the same time there is a marked
reduction in gNa+. This coupled with the substantial increase in
K+
outflow is sufficient not only to eliminate the internal
positivity caused by the Na+
inflow, but also to actually
restore the original resting membrane potential.
It is
important to understand that the depolarization caused by the
Na+
inflow and the repolarization caused by the K+
outflow
occur locally. That is, they occur only on that section of axon
which is initially stimulated. The entire action potential,
including depolarization to the reversed potential and repolarization back to the resting membrane potential, happens
very quickly, requiring no more than a few milliseconds.
 The Action
Potential Involves a Very Small Transfer of Ions
The Action
Potential Involves a Very Small Transfer of Ions
The
capacitance of a typical nerve cell membrane has been estimated
to be 1 µF/cm2 Therefore
the number of charges which need to be transferred across the
membrane capacitor to change its potential by 125 mV is given
by
Q=CV
= (10-6 F/cm2) (1.25 x 10-1 V) . = 1.25
X 10-7C/cm2
Now the
number of sodium ions which need to diffuse inward in order to
transfer 1. 25 x
10-7C of charge from the extracellular fluid,
through one square centimeter of membrane, to inside can be
calculated from the Faraday constant.
Number of
moles transferred per square centimeter = membrane charges transferred
per square centimeter x
(1/ Faraday constant )
= (1.25
X 10-7C/cm2) (1 x 10-5 mol/C)
= 1.25 x
10-12 mol/cm2
= 1.25 pmol/cm2
These few
picomoles which diffuse inward during the depolarization of the
membrane to the reversed potential stage are so insignificantly
few in a largediameter nerve fiber that they cause virtually no
change in the measurable extracellular or intracellular sodium
concentrations. Similarly, the outward diffusion of 1.25 pmol
of K+
per square centimeter is sufficient to repolarize the
membrane back to the resting level, and yet this loss of
intracellular K+
is so insignificant as to leave virtually
unchanged the extracellular and intracellular K+
concentrations. Of course, the smaller the fiber the greater
will be the change in the intracellular concentrations of these
ions. But still the change would be insignificantly slight. In
any event, the nerve cells are constantly being recharged by
active transport outward of the few picomoles of Na+
which
diffuse inside during depolarization, and by actively
transporting inward the few picomoles of K+
which diffuse
outward during depolarization. Recharging enables neurons to
conduct virtually unlimited numbers of impulses without
producing changes in the ionic concentrations which are vital
for maintaining their excitability.
As a
theoretical exercise you can calculate the small percentage of
internal K+
which needs to diffuse outward in order to repolarize the membrane by considering the axon to be a
cylinder of uniform diameter. For example, consider a large
mammalian nerve axon with a diameter of 20
µm (2 x 10-3 cm)
and an intracellular concentration of K+
equal to 140 mmol/L.
Percent of
intracellular K+
diffusing out = (number of
moles of K+
diffusing out through a given length of axon / number of moles
of K+
in axoplasm for a given
length of axon) x 100
=
{[(moles diffusing out)/(cm2 of membrane)](cm2
of membrane) / (mol/cm2
of axoplasm)} x 100
= {(1.25 x
10-12 mol/cm2) [277(1 X 10-1 cm)] (cm) / (1.4 X 10-4
mol/cm2) [77(1 x 10-1 cm)2](cm)} x 100
=(7.9
x 10-15 mol / 4.4 X 10-10
mol ) x 100
= 1.8 x
10-3 percent
During the
remainder of our discussion on the events associated with the
action potential we will examine exclusively the work done with
the giant axon of the squid. You really lose nothing by
abandoning for the moment the mammalian axon in favor of
concentrating on the squid axon. In fact. quite to the contrary,
you get a real feel for the work of Hodgkin and Huxley in
developing the principles we accept so easily today.
 Sodium and
Potassium Conductance
Sodium and
Potassium Conductance
Using a
technique called the voltage clamp, Hodgkin and Huxley
calculated the time course of sodium and potassium conductance.
g"o~ and gl(~' as well as sodium and potassium current, I"" T
and I I( ~, during an action potential. We will examine this
technique later, but for the moment consider the calculated
relationships with time during the action potential illustrated
in Fig-12.
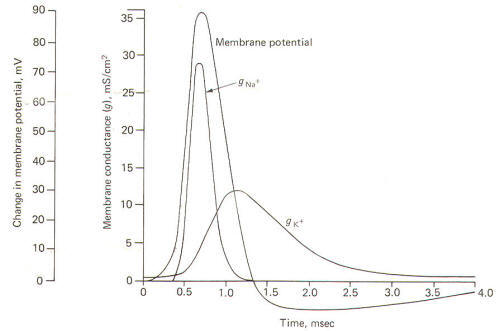 |
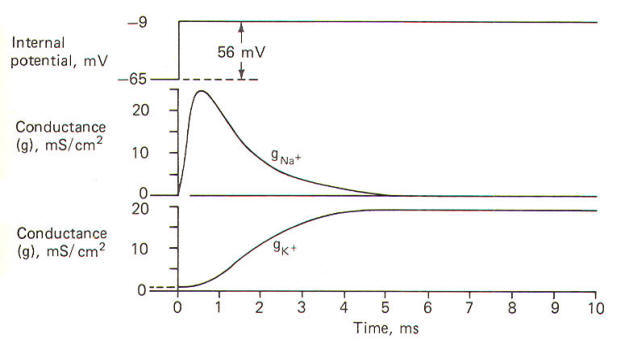
| Fig-12 |
The action
potential pictured here underwent a sudden but reversible change
in its membrane potential. Notice that the initial value of the
RMP and the final value of the reversed potential are not
indicated. Instead all we see is the magnitude of depolarization
from the former to the latter. Notice that when the membrane
potential has depolarized by about 10 mV (presumably to the
excitation threshold) there is a sudden and large increase in
gNa+ to about 30 mS/cm2 This is responsible for the large and
sudden change in the membrane potential. Notice further that
this large increase in g"" - is transient and the conductivity
returns within a few milliseconds to practically zero. Meanwhile
a slower increase in the
gK+ to about 12 mS/cm2, which started
even before the membrane potential reached its maximum reversed
potential. promotes the repolarization of the membrane.
Consequently the membrane potential changes once again back to
the resting level. In fact the membrane often
hyperpolarizes beyond the resting level by a few millivolts
before gradually returning to the resting level after several
milliseconds. This slow return to the resting level is called
the afterpotential. The rapid rise and fall in membrane
potential is called the spike or spike potential. However, the
action potential includes both the spike potential and the afterpotential.
 The Impulse
The Impulse
During the
reversed potential the axoplasm immediately inside the
stimulated area of membrane is temporarily made positive while
the adjacent axoplasm is still negative. Similarly the
extracellular fluid immediately outside the stimulated area is
temporarily negative while the adjacent extracellular fluid is
still positive. Thus charge gradients exist side by side and a
small current begins to flow in a circuit through the membrane.
The direction of this current is inward through the depolarized
areas, laterally through the adjacent axoplasm, outward through
the adjacent still-polarized membrane segment immediately
adjacent to the depolarized area, laterally backward through
the extracellular fluid, and inward once again through the
depolarized area. The path of this local current is illustrated
in Fig-13.
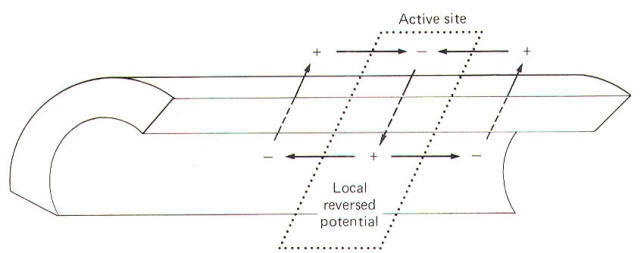 |
| Fig-13 |
As the
local current flows outward through the adjacent still-polarized
membrane, the membrane at this point begins to depolarize. Once
it has depolarized to the excitation threshold, the sodium
channels suddenly open and the resultant increase in
gNa+
causes the now-familiar action
potential to occur at that point on the membrane. Subsequently,
as this new action potential develops, a new local current will
flow from it to the next adjacent membrane segment,
depolarizing it and propagating a continuous impulse down the
axon.
Of course
if the axon is stimulated at some point along its length the
local current will spread in both directions away from the
stimulus site and an impulse will travel in both directions.
You should always keep in mind that this condition (impulse
spread in both directions) occurs only in the laboratory
preparations when neurons are stimulated along their axons. As
we pointed out earlier, neurons are rarely stimulated on their
axons in vivo. Instead they are stimulated in dendritic zones to
produce action potentials via generator potentials from sensory
receptors and neurotransmitters from presynaptic terminals at
synapses. In axons, local currents traveling ahead of propagated
action potentials are responsible. In all these naturally
occurring stimulus situations, the local currents and hence the
propagated action potentials travel in only one direction. This
direction is toward the terminal branches of the axon.
Approximately 0.5 ms after the local area of membrane
depolarizes, it starts to repolarize as a result of the
progressive increase in
gK+ . Thus the impulse which travels
down an axon is followed about 0.5 ms later by a wave of
repolarization as each succeeding membrane segment begins to
repolarize.
 Propagation
of Action Potentials in Myelinated Neurons
Propagation
of Action Potentials in Myelinated Neurons
Myelinated
neurons propagate action potentials with the same kinds of ion
movements as the nonmyelinated neurons just described. The
fundamental difference is that the local current flows through
the membrane only at the nodes of Ranvier. These nodes are the
interruptions in the sheath which surrounds the axons of all
myelinated neurons. Current flows through the membrane only at
these nodes because they represent areas of relatively low
electrical resistance, while the myelinated internodes offer
relatively high resistance to current flow. Consequently, when
a myelinated neuron is stimulated and an action potential is
generated, the local current which flows through the adjacent
axoplasm will pass out through the first node rather than
through the next adjacent area of membrane. A comparison of the
nature of local current flow in myelinated and nonmyelinated
axons is illustrated in Fig-14.
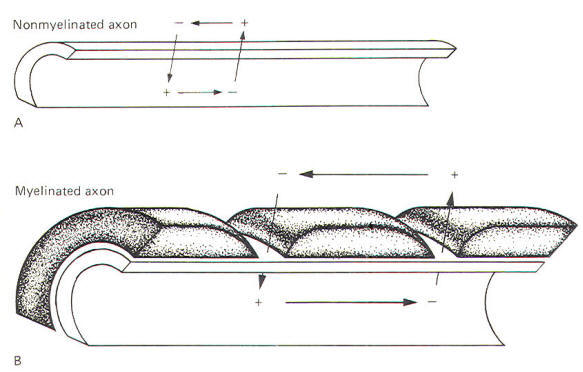 |
| Fig-14 |
In a
nonmyelinated neuron the local current must depolarize each
adjacent area of membrane - a relatively time-consuming process.
Since the impulse proceeds only as rapidly as the spread of the
local current, this need to depolarize each adjacent area of
membrane imposes necessary restrictions on the conduction
velocity. Myelinated neurons, on the other hand, have an
advantage which enables them to conduct impulses with a much
higher velocity. Since the local current does not need to
depolarize each adjacent area of the membrane, impulses travel
along the axon at a greatly accelerated velocity. This is called
saltatory conduction.
 Fiber
Diameter and Conduction Velocity
Fiber
Diameter and Conduction Velocity
Conduction
velocity is roughly proportional to fiber diameter. The greater
the diameter of the axon, the greater the conduction velocity.
This is true because the larger the diameter, the greater the
cross-sectional area of the axoplasm and hence the lower its
electrical resistance. Thus a large local current will spread
further along the axoplasm before flowing outward through the
membrane to complete the circuit. Consequently a greater length
of axonal membrane will be depolarized faster, and action
potentials will be propagated at a greater velocity. If we
think of a length of axon as having a uniform cross-sectional
area, the internal axoplasmic resistance to current flow can be
calculated using the same assumptions underlying resistance in a
length of wire.
Ri= ri /π
(radius)2
where Ri= axoplasmic resistance per unit length of axon,
Ω /cm ri = axoplasmic resistivity,
Ω /cm radius =
radius of axon, cm
 THE LOCAL
CURRENT: A CLOSER EXAMINATION
THE LOCAL
CURRENT: A CLOSER EXAMINATION
An
important point to consider is that one action potential cannot
propagate a second without the contribution of the local
current. Thus it is clear that the local current plays a crucial
role in the impulse conduction process. Current
flow in the axon has been likened to current flow in a large
undersea cable. Both are composed of a long conducting core
(the axoplasm in the neuron) surrounded by an insulator (the
neuronal membrane) and immersed in a large-volume conductor (the
neuronal extracellular fluid). The axon, however, behaves as a
leaky cable in that current not only flows through the axoplasm
but leaks out through the membrane as well. Since the same
electrical rules apply to current flow in the cable and in the
axon, neurophysiologists often speak of the cable properties of
the axon.
The current
which spreads with the impulse is an active current. The local
current which we have been discussing is, by contrast, a passive
current, and its spread depends only on electrical parameters of
the conducting material such as the resistance and capacitance
of a unit length of axon. These passive or cable properties of
the axon determine the extent and magnitude of the local
current.
The local
current spreads only a very short distance through the axoplasm
before flowing out through the membrane, partially depolarizing
it, and producing an electrotonic potential (Fig-19). Electrotonic potentials can be observed only when the degree of
stimulation is subthreshold because once the excitation
threshold is reached the small electrotonic potential is
obliterated by the large potential changes associated with the
much larger action potential.
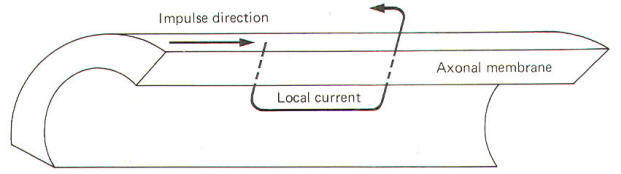 |
| Fig-15 |
The
electrotonic potential is the difference between the
subthreshold membrane potential at any given time and the
resting membrane potential. As action potentials are propagated
down the axon, local currents can be visualized as preceding
them, depolarizing each newly encountered resting membrane
segment and establishing electrotonic potentials which reach
threshold and produce additional action potentials (Fig-15).
 Electrical
Properties of the Membrane and Surrounding Fluids
Electrical
Properties of the Membrane and Surrounding Fluids
It is often
helpful to picture the membrane and surrounding fluids as an
electrical circuit in order to understand the local current
flow and the electrotonic potential which it produces. Researchers alike are indebted to the work of Hodgkin for
our electrical models of the axon. He pictured the membrane as
composed of an infinite number of electrotonic "patches" with
each patch composed of a resistance and capacitance in parallel
surrounded by intracellular and extracellular fluids, both
offering series resistance to the flow of the local current
(Fig-16).
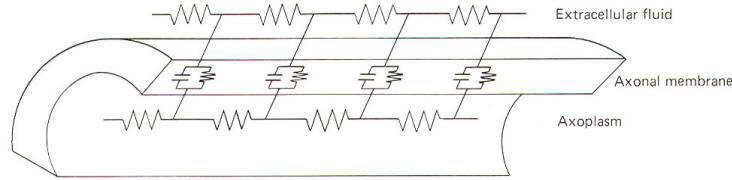 |
| Fig-16 |
 Ionic and
Capacitive Current
Ionic and
Capacitive Current
Electrically, the membrane can be thought of as a resistance and
capacitance in parallel. The membrane resistance RM represents
the difficulty encountered by ions in diffusing through their
respective membrane channels, while the membrane capacitance CM
represents the charge which exists across the membrane at any
time. Now remember that current flow in biological systems
consists of moving charges carried by ions. Therefore both
capacitive and ionic currents represent the flow of ionic
charge. Ionic current II is the charge carried by ions as they
flow through their respective ionic channels in the membrane.
The difficulty they encounter in passing through these channels
from one side of the membrane to the other is represented by the
membrane resistance RM . Capacitive current Ic, on the other
hand, does not represent the actual flow of ions through the
membrane. Its explanation is a little more subtle. If positive
ions flow through the axoplasm to the inside of the membrane
they will neutralize some of the negative ions already there.
This will free some of the positive ions from the immediate
membrane exterior to flow away since they are no longer held to
the membrane capacitor. Thus positive ions have moved up to and
away from the membrane. Thus current has, in effect, traveled
outward through the membrane even though no actual ions have
crossed from one side to the other. Remember that capacitive
current Ic flows only while a capacitor is being charged or
discharged. Ionic and capacitive currents are illustrated in
Fig-17.
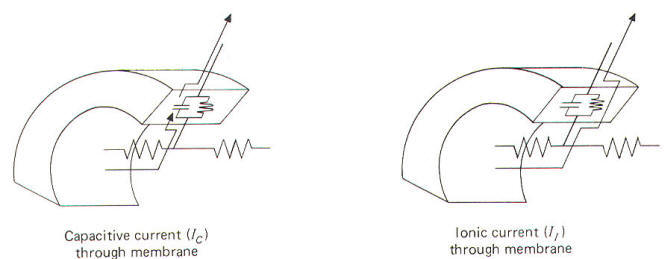 |
| Fig-17 |
 The Nature
of the Local Current
The Nature
of the Local Current
When the local membrane site undergoes an action
potential, a local current flows which establishes an
electrotonic potential on the next adjacent area of membrane.
When this electrotonic potential reaches the excitation
threshold, gNa+ will suddenly increase and a second action
potential will be generated, obliterating the electrotonic
potential and the local current. Unfortunately for
experimenters, action potentials propagate so quickly that there
is not sufficient time to study the local current itself.
However, if the membrane is stimulated to a subthreshold level
and held there, the nature and time course of the local current
can be studied.
A
convenient way to do this is to penetrate an axon with a
depolarizing microelectrode. In this way a steady but
subthreshold level of depolarizing (positive) current can be
released internally. By keeping the level of stimulation steady
and subthreshold, and by recording the potential changes of the
membrane at various distances from the stimulating site, one
can examine the magnitude, distance, and time course of the
local current spread. Consider the membrane circuit pictured in
Fig-18. A depolarizing microelectrode has been placed into
the axoplasm at patch A while recording microelectrodes have
been inserted at patches B, C, and D. Assume that a steady
subthreshold depolarizing current is applied at patch A. A local
current will now flow from the less negative region near the tip
of the depolarizing microelectrode to the still polarized (more
negative) regions of axoplasm at patches B, C, and D before
flowing out through the membrane to complete the circuit.
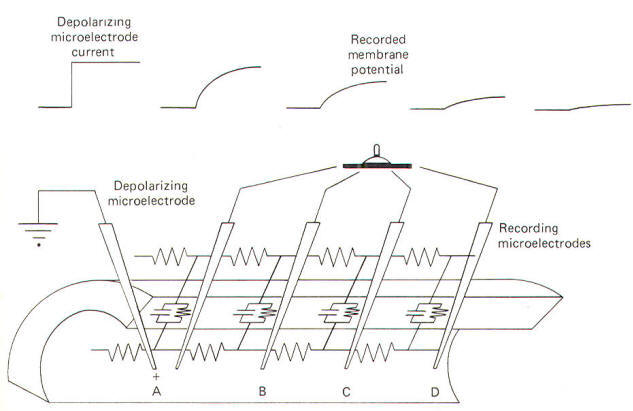 |
| Fig-18 |
From
previous discussions we know that current flow through the
membrane is both capacitive and ionic. Both kinds flow in the
above circuit. When current is first applied to the axoplasm at
patch A, most of it initially goes toward
discharging the CM at patch A. Hence Ic flows outward through
the membrane at patch A. Initially no II flows outward through
patch A because there is no net driving force across the
membrane. But as the transmembrane potential is altered from its
resting level (RMP) and an electrotonic potential is developed,
a net driving force is built up across the membrane. Once the
membrane capacitor at patch A has been charged up to the level
of the steady depolarizing current at patch A, Ic stops and
subsequent current flow outward through the membrane at patch A
is purely ionic (II). Not all current from the depolarizing
microelectrode becomes outward Ic and II at patch A. Some,
progressively less, continues to flow through the internal
axoplasmic resistance Ri to first become capacitive and then
ionic current through membrane patches B, C, and D.
Because
voltage drops (decreases) as current flows through increasingly
distant lengths of axoplasmic resistance, the membrane capacitors
at patches B, C, and D are progressively less completely
discharged and exhibit smaller and smaller electrotonic
potentials. This is illustrated by the progressively decreased
voltage changes recorded by electrodes at patches B, C, and D.
Remember that current follows the path of least resistance;
therefore, most of the current flows out through the membrane at
patch A with progressively less reaching and subsequently
traversing more distant points on the membrane. At sufficiently
great distances, beyond the reach of the local current, no
electrotonic potential is established and the resting membrane
potential remains undisturbed.
 Axon
Geometry and the Local Current
Axon
Geometry and the Local Current
The
electrotonic potential decreases exponentially with distance
from the active (stimulated) site according to a value which is
known as the length constant λ.
λ
= [RM/ (Ri +
Re)]1/2 where
λ =
length constant [the distance over which the electrotonic
potential decreases to 1/e (37 percent) of its maximum value],
cm
RM =
membrane resistance per unit length of axon,
Ω.cm
Ri =
axoplasmic resistance per unit length of axon,
Ω/cm
Re =
extracellular fluid resistance per unit length of axon,
Ω/cm
Because of
the relatively large volume of the extracellular fluid, its
resistance to current flow is very small and can effectively be
removed from the above equation, leaving the simple relationship
below.
λ = (
RM/ Ri)1/2
The length constant is a measure of
how far the local current spreads along the axon in front of the
action potential. Remember that action potentials give rise to
local currents in vivo, while in the experimental situation just
described, the depolarizing microelectrode gave rise to the
local current. In any case, the longer the length constant the
farther the local current will spread through the axon,
producing progressively smaller electrotonic potentials before
it dies out.
Let's now
examine those factors which determine the values of
RM and
Ri
since these determine the value of the length constant. If we
think of a length of axon as having a uniform cross-sectional
area, we can calculate Ri as follows:
Ri
= ri /π(radius)2
where
Ri =
axoplasmic resistance per unit length of axon,
Ω/cm ri =
axoplasmic resistivity, Ω.cm radius =
radius of axon, cm
The
transverse resistance through the membrane for a given length of
axon is:
RM =
rM / 2π(radius)
where
RM =
membrane resistance per unit length of axon,
Ω/cm rM
= specific
membrane resistance, Ω/cm2
Notice that
increasing the radius of the axon decreases both the
Ri and
RM
but there is a greater proportional decrease in
Ri . Consequently
the length constant increases with axon diameter. A long length constant means that
larger segments of adjacent membrane will be depolarized faster.
Thus as, already pointed out, the larger the axon
diameter, the greater the impulse conduction velocity. Most of
the aspects of the local current are illustrated in Fig-19.
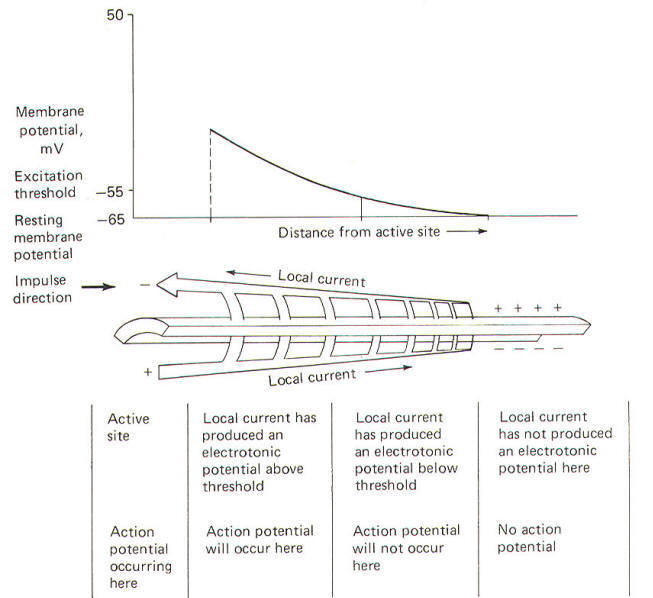 |
| Fig-19 |
 THE VOLTAGE
CLAMP EXPERIMENTS OF HODGKIN AND HUXLEY
THE VOLTAGE
CLAMP EXPERIMENTS OF HODGKIN AND HUXLEY
You should
be thoroughly familiar now with the relationship between the
local current and the action potential. You should also be aware
that when the action potential is initiated, several membrane
variables change rapidly as a function of time. These are
potential, conductance, and current. Now remember that the Ohm's
law relationship between them is expressed by:
g=I/V where
g
= conductance, S
I = current,
A V =
potential, V
Examination
of this relationship shows that g varies as a function of
I and
V. Hodgkin and Huxley set out to determine the changes in
membrane conductance
gM
during the action potential. Their problem was
that both the membrane current lM and the membrane potential
VM are also changing constantly during the action potential.
Now if one of these variables could be held constant during the
action potential (i.e., VM), measurement of lM
would enable them
to calculate
gM at any instant. This is what the voltage clamp
technique enabled them to do. A voltage clamp setup is
diagrammatically illustrated in Fig-20.
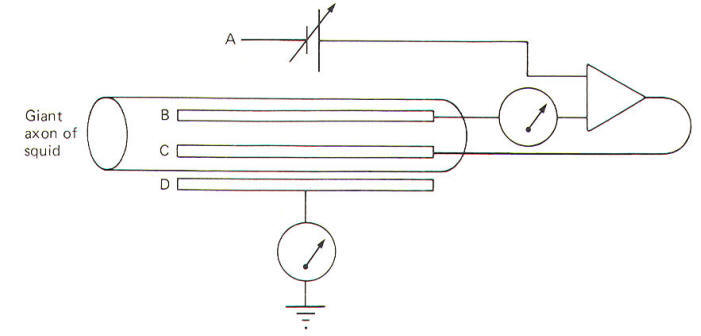 |
| Fig-20 |
The system
operates like this. The experimenter decides on a voltage he
would like
to produce across the membrane and then sets this "command
voltage" on an external voltage source at A (VA). The voltage
recording microelectrode at B detects whatever voltage
presently exists across the membrane (VB) and sends this signal
into the differential amplifier. The signal from the command
voltage source is also transferred into the differential
amplifier. Now a differential amplifier produces no output if
the voltages on its two inputs are equal (VA =
VB). But if they
are not equal (VA
≠ VB), the amplifier will send whatever
current is necessary into the intracellular current-passing
electrode at C in order to change the membrane voltage recorded
by the recording microelectrode at B until it equals the
command voltage. As soon as VA equals VB, the amplifier stops
its output.
The
differential amplifier effectively alters the voltage across the
membrane by sending a current through the membrane from the
current-passing electrode to the current-recording electrode at
D. In the course of crossing a membrane, current
discharges the membrane capacitor and hence the membrane
voltage. This altered voltage is sent to the differential
amplifier for comparison with the command voltage. Consequently
the experimenter can "dial" any desired voltage across the
membrane, and even more importantly, hold the membrane potential
at that level.
Now let's
examine an experimental situation. Suppose that the RMP of a
giant squid axon is -65 mV and the experimenter wishes to
"clamp" the voltage at -9 mV. The command voltage is first set
at -9 mV. Within microseconds of applying the command voltage,
the differential amplifier will pass sufficient current through
the membrane to lower the RMP by 56 mV to the set level of -9 mV. Since the membrane potential has now greatly exceeded the
excitation threshold, the Na+
channels open, but because of the
voltage clamp no actual change in membrane potential is
observed. Nevertheless, Na+
ions diffuse inward down their
chemical gradient. As they do so, the differential varies its
current output proportionally to prevent the Na+
inflow from
altering the condition which the amplifier is designed to
preserve (VA = VB). Slightly later the K+
channels open and
the differential amplifier once again varies its current output
proportionally to prevent the K+
outflow from altering the
clamped condition
(VA =
VB).
It only
takes a few microseconds for the voltage clamp to fix the
membrane potential at -9 mV once the command voltage
is first applied. Therefore, any subsequent current changes
detected by the current-detecting electrode (D) are in response
to, and in the opposite direction from, any ionic currents
crossing the membrane with Na+
inflow and K+
outflow.
The results
obtained by Hodgkin and Huxley when they depolarized the
membrane of the squid giant axon by 56 mV are pictured in Fig-21. Notice that the membrane current
IM is first inward
(presumably carried by Na+) and then outward (presumably carried
by K+).
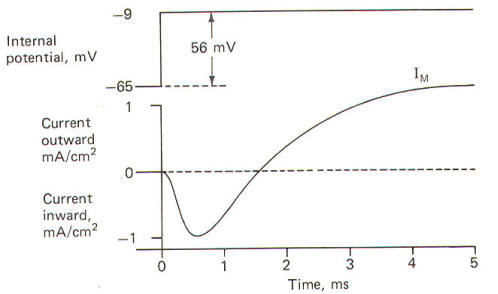 |
 |
| Fig-21 |
Fig-22 |
 Sodium and
Potassium Currents
Sodium and
Potassium Currents
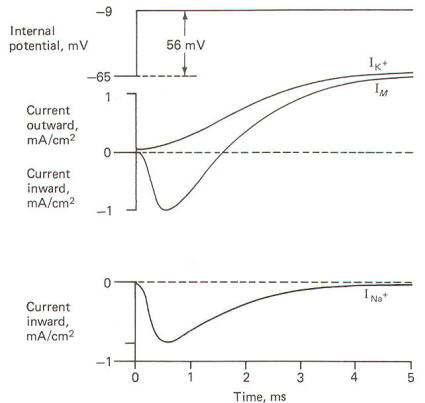
When the
squid giant axon is bathed by seawater, a solution similar to
its extracellular fluid, and then stimulated to its excitation
threshold, the IM is first directed inward and then outward
(Fig-22). The contribution of Na+
to the IM could
conceivably be eliminated if the extracellular Na+
were reduced
to the level of the axoplasm, as this would eliminate the
chemical gradient which powers the inflow. Hodgkin and Huxley
did this and obtained the results in Fig-22. Notice that
following the reduction of the extracellular Na+
to axoplasmic
levels, the current flow following stimulation only has an
outward component. This current is presumably due almost
exclusively to K+
outflow and represents the potassium current
IK+. Accordingly, the sodium current INa+ is calculated by
subtracting the IK+ from the IM. Presumably
IM = IK+
-
INa+.
Once they
had recorded individual ionic currents against a fixed voltage,
it was a simple matter using Ohm's law to mathematically
calculate individual ionic conductances and then plot them as a
function of time. They developed the following equations to do
this and then plotted the results in Fig-23.
 |
| Fig-23 |
gion=
Iion /(VM - Eion
)
gK+
=IK+
/(VM-EK+)
gNa+
=INa+/(
VM-ENa+)
where
gion
= ionic conduction, µmS/cm2
Iion = ionic current, mA/cm2
VM =
membrane potential, mV Eion =
ionic equilibrium potential, mV
 Electrical
Components of the Action Potential
Electrical
Components of the Action Potential
One action potential propagates a second by
means of the local current. The local
current depolarizes the adjacent membrane to the excitation
threshold at which point rapid but reversible changes occur in
gNa+,
INa+, and
IK+, resulting in the familiar action
potential. Fig-24 illustrates the time course of all these
events occurring at a single finite location as they were
calculated by Hodgkin and Huxley for the data they collected
with the voltage clamp experiments.
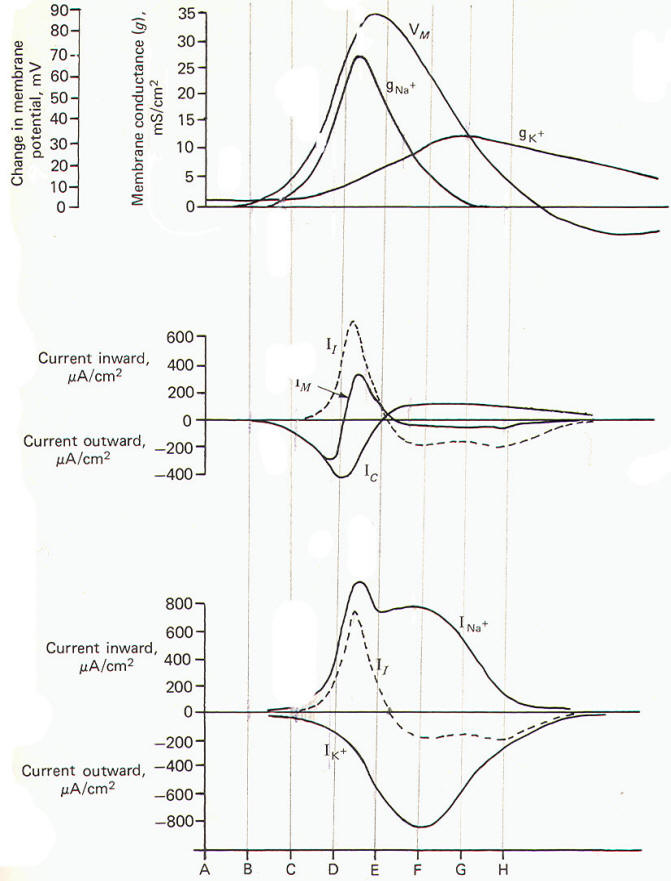 |
Now let's
examine the various electrical changes which occur in one single
axon location as the impulse arrives at this location, passes
over it, and then proceeds down the length of the axon. Using
Fig-24 we will summarize the changes during eight
instantaneous time segments beginning with the resting membrane
before the arrival of the impulse.
A The
membrane is in the resting state since the approaching impulse
has not yet reached this point on the axon.
gK+ is greater than
gNa+ and
IM is small.
B The
approaching impulse is closing in on the local axon section and
the local current traveling in front of it is starting to
depolarize the membrane and causing an initial outward Ic.
gK+
is still greater than
gNa+. The
lc accounts for all of the IM
at this time.
C The
outward lc caused by the local current has depolarized the
membrane by about 10 mV to the excitation threshold. Na+
channels are opening so that inward Na+
and outward K+
diffusion is equal. Thus INa+ and
IK+ are temporarily equal
and opposite. This is an unstable condition and the membrane is
at threshold.
D The
gNa+
is now considerably greater than
gK+ and the inward
INa+ now
exceeds the outward IK+ and is responsible for the overall
inward direction of the II. The II is discharging the membrane
capacitor as it flows inward depolarizing the membrane.
E The
membrane is at the peak of its depolarization, having
established its maximum reversed potential. The
gNa+
and INa+ have begun to decrease while the
gK+ and
IK+ have begun to
increase. The INa+ and
IK+ are equal and opposite.
F The
gNa+ has decreased to where it equals the increasing
gK+. But
now the outward IK+ exceeds the inward
INa+ and thus the If is
directed outward. This is countered by an opposite but less than
equal inwardly directed Ic. Hence the IM is now directed
outward.
G The
gK+
now greatly exceeds the
gNa+
and the IK+ still exceeds the
INa+· Thus the
II is still directed outward. Since the outward
II still
slightly exceeds the inward Ic, the IM is small, but still
directed outward. The membrane continues to repolarize.
H The
gK+
and IK+ are still greater than their resting levels, while the
gNa+ is now even lower than its resting level. Hence the
membrane potential VM is driven toward the potassium
equilibrium potential EK+ producing the hyperpolarized
afterpotential. |
| Fig-24 |
|
|